MRNA stock is lower after hours because the FDA delivered a hard stop on a program that investors had treated as one of Moderna's most realistic "next product" bridges beyond its COVID franchise.
The agency's Center for Biologics Evaluation and Research (CBER) issued a Refusal-to-File (RTF) letter for Moderna's biologics license application (BLA) for its investigational seasonal influenza vaccine, mRNA-1010. It also said it would not initiate a review.

Despite that, Moderna says the letter did not raise safety or efficacy concerns about the vaccine itself, and that the issue is tied to the trial comparator used in its Phase 3 efficacy study.
For traders, that is enough to move the stock quickly. When a key product timeline becomes less clear, markets tend to reprice risk fast, especially in extended trading when liquidity is thinner.
What Happened to MRNA Stock After Hours?
| Item |
Level / Detail |
| Regular-session close |
~$42.00 |
| After-hours print (example) |
~$38.27 (-8.88%) |
| Day range |
~$41.68 to ~$45.50 |
| 52-week range |
~$22.28 to ~$55.20 |
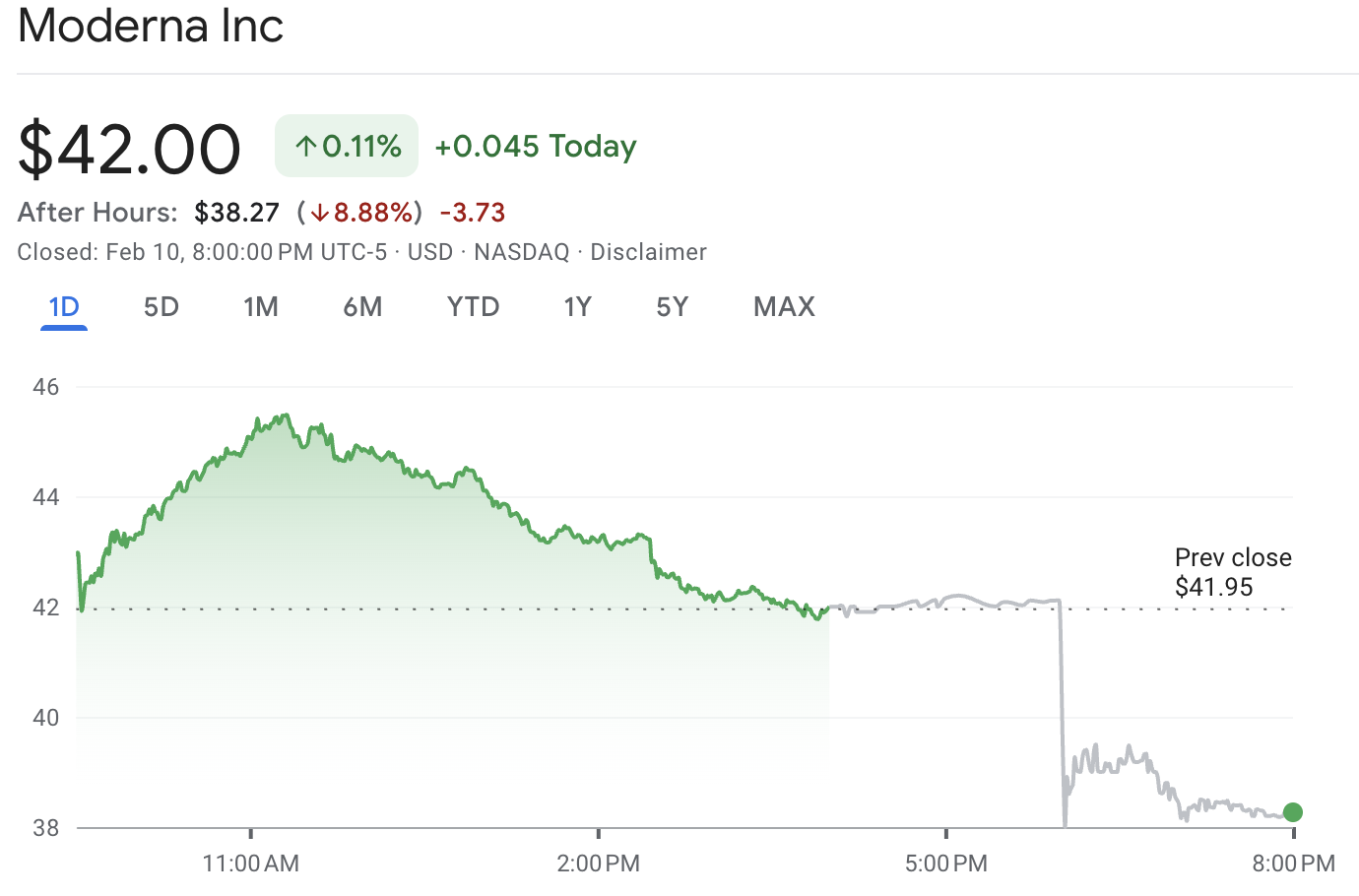
MRNA stock closed at approximately $42.00 before dropping to about $38.27 in after-hours trading, representing a decline of around 8.9% at the time of the report.
What Was the Main Catalyst?

The FDA Refused to File Moderna's Flu Vaccine Application
Moderna announced that it received a letter from the FDA's Center for Biologics Evaluation and Research (CBER), stating that it will not initiate a review of the mRNA-1010 Biologics License Application (BLA) and will instead issue a Refusal to File (RTF) letter.
The company highlighted that it had utilized a Priority Review Voucher to help ensure a timely review. This rejection is particularly disappointing for Moderna from a market perspective, as it suggests that the issue is not merely a matter of being "in the queue."
Additionally, Moderna indicated that this refusal contradicts the feedback it received during pre-Phase 3 and pre-submission consultations. Consequently, the company has requested a Type A meeting to clarify the path forward.
What Is a Refusal-To-File Letter?
An RTF is a formal tool the FDA uses to avoid spending time reviewing applications that it considers incomplete or not suitable for a meaningful review at the filing stage.
The FDA's internal procedure defines RTF as a method to prevent the unnecessary review of incomplete applications. It highlights that an RTF action allows applicants to address "critical insufficiencies" before submitting a complete application, which can then undergo substantive review.
Two practical points matter for traders:
An RTF is not the same as a full approval or rejection after a complete review.
The path forward hinges on the FDA's requested changes and the company's ability to respond swiftly.
Why the FDA Said No to Moderna's Flu Vaccine?

The central issue, as described across multiple reports, is that the FDA did not view Moderna's study design as "adequate and well-controlled" because the comparator arm did not reflect the FDA's view of the best available standard of care, particularly for older adults.
According to reporting, Moderna compared mRNA-1010 against a standard-dose licensed flu vaccine (often cited as GSK's Fluarix in coverage).
At the same time, the FDA signaled it expected a comparator that better matches how high-risk populations, especially those 65 and older, are commonly vaccinated in the United States (often with enhanced or high-dose options).
Moderna disputes the move. In its statement, the company said the RTF did not cite safety or efficacy issues and described the decision as inconsistent with earlier FDA feedback.
Moderna's Key Claims (From Its Release)
The FDA cited the standard-dose comparator as the sole reason for refusing to initiate review.
Moderna stated the FDA did not identify specific safety or efficacy concerns.
Moderna requested a Type A meeting with FDA leadership to clarify a path forward.
Moderna stated that it does not expect this decision to impact its 2026 financial guidance.
Why the Comparator Issue Is Critical?
In vaccine trials, the comparator is the "baseline" product used to judge the new product's performance. If the regulator believes the comparator does not represent the right real-world standard, the regulator may argue that the evidence does not answer the question it needs answered.
That is why markets react strongly even when the company says there is no new safety concern. The dispute shifts the debate from "Does it work?" to "Does the evidence meet the FDA's standard for this population right now?"
This also explains why traders often treat RTF headlines as "timeline events." The vaccine may still reach the market, but the timing becomes less predictable.
What This Means for Moderna's Flu Timeline
Moderna announced that mRNA-1010 is currently under review in the EU, Canada, and Australia. The company anticipates that the earliest approvals in these regions will occur in late 2026 or early 2027, based on current evaluations.
In the United States, the next steps will depend on the outcome of the Type A meeting. This will determine whether Moderna needs to resubmit the Biologics License Application (BLA) with additional data, a different approach, or revised framing that meets the FDA's expectations for comparators.
Why Was MRNA Stock Reaction So Fast: It Reopens a Familiar Moderna Concern
MRNA has been trying to convince investors that its post-COVID future is built on a portfolio, not on a single product. Flu is central to that narrative because seasonal respiratory markets are large, recurring, and commercially proven.
This FDA refusal forces a reset on three fronts.
1) It Pushes Out a Near-Term Launch Pathway in the U.S.
Even if the issue is solvable, an RTF means Moderna does not have an active FDA review underway today. That alone reduces near-term visibility and increases the gap between "data" and "revenue."
2) It Increases Uncertainty Around Moderna's Combo Respiratory Strategy
Moderna has withdrawn its FDA filing for the mRNA-1083 flu and COVID combination vaccine after the FDA requested more data on flu efficacy before proceeding with the review. That history matters because the market has already watched Moderna's respiratory timeline slip once.
3) It Signals a Stricter Bar for Respiratory Vaccine Comparators
If the FDA is now emphasizing "best available standard of care" comparators for older adults, that is not a one-program issue. It can shape how companies design future influenza trials and how quickly they can iterate.
Why This Headline Can Bleed Into the Next Earnings Reaction
MRNA's next scheduled earnings date is on February 13, 2026, and options pricing anticipates a weekly implied move of about 11.63% into that event window.
An implied move above 10% usually tells you that investors expect meaningful uncertainty around headlines, guidance, or both.
After an FDA refusal-to-file event, the market often does two things:
It demands clearer language on the path forward and timelines.
It reprices implied volatility if it expects follow-up communication, such as an FDA meeting outcome, to land near the same time window.
MRNA Stock Technical Analysis: Levels Traders Should Watch Tonight
| Indicator |
Value |
Signal |
| RSI (14) |
48.155 |
Neutral |
| MACD (12,26) |
0.29 |
Buy |
| ATR (14) |
0.9374 |
High volatility |
| ADX (14) |
40.829 |
Sell |
MRNA's daily technical posture was broadly neutral, with mixed signals across indicators and moving averages at the time of the after-hours move.
Pivot Levels (Classic)
| Level type |
Price level |
How traders often use it after a headline |
| Classic S1 |
$41.74 |
First area where dip buyers may test if the tape stabilizes. |
| Classic S2 |
$41.31 |
A break below increases odds of a retest of deeper supports. |
| Classic S3 |
$40.51 |
A key "line" because it sits near longer-term moving average congestion. |
| Classic Pivot |
$42.54 |
Regaining the pivot often signals the market is fading the panic. |
| Classic R1 |
$42.97 |
First upside checkpoint if the stock rebounds into earnings. |
| Classic R2 |
$43.77 |
A stronger rebound zone that often requires fundamental clarification. |
How to Read This Setup:
If the stock holds above the low-$40 area during normal hours, it suggests buyers are still defending the trend.
If weakness from after-hours persists into the next session and the price remains below key moving averages, market sentiment may remain fragile until new information emerges.
Frequently Asked Questions (FAQ)
Why Is MRNA Stock Down After Hours Today?
After hours, MRNA stock fell following Moderna's announcement that the FDA issued a refusal-to-file letter for the mRNA-1010 influenza vaccine BLA, indicating that the FDA will not initiate a review.
Did the FDA Say Moderna's Flu Vaccine Is Unsafe?
No. Moderna's statement indicates the refusal-to-file letter did not identify specific safety concerns about mRNA-1010.
Why Did the FDA Focus on the Comparator Vaccine?
Because the comparator is the benchmark for judging performance, and the FDA said the comparator used did not reflect the "best-available standard of care," particularly for older adults.
Does Moderna Expect This to Change Its 2026 Guidance?
Moderna said it does not expect any impact on its 2026 financial guidance from the refusal-to-file decision. Investors still sold the stock because the flu program's U.S. timing is important for longer-term revenue visibility.
Can Moderna Still Get Approval Outside the United States?
Yes. Moderna announced that the vaccine has been accepted for review in the EU, Canada, and Australia, with potential approvals expected to begin in late 2026 or early 2027.
Conclusion
In conclusion, MRNA stock is down after hours because the FDA issued a refusal-to-file letter for Moderna's mRNA flu vaccine application, which raises near-term uncertainty around the U.S. timeline.
The key point is that this move is about regulatory process and trial design standards, not a newly disclosed safety problem.
The next swing factor is not a debate about influenza immunology. The next swing factor is whether Moderna can secure a clear path forward through an FDA Type A meeting and whether that path requires new clinical work. Until that clarity arrives, MRNA stock is likely to trade as a headline-sensitive, high-volatility name into its next earnings window.
Disclaimer: This material is for general information purposes only and is not intended as (and should not be considered to be) financial, investment or other advice on which reliance should be placed. No opinion given in the material constitutes a recommendation by EBC or the author that any particular investment, security, transaction or investment strategy is suitable for any specific person.





























