A retest is possible if the offering prices cleanly, the FDA path is confirmed, and confidence holds in AMT‑130's 36‑month efficacy, while dilution and regulatory scrutiny are the near‑term brakes.
Market Move
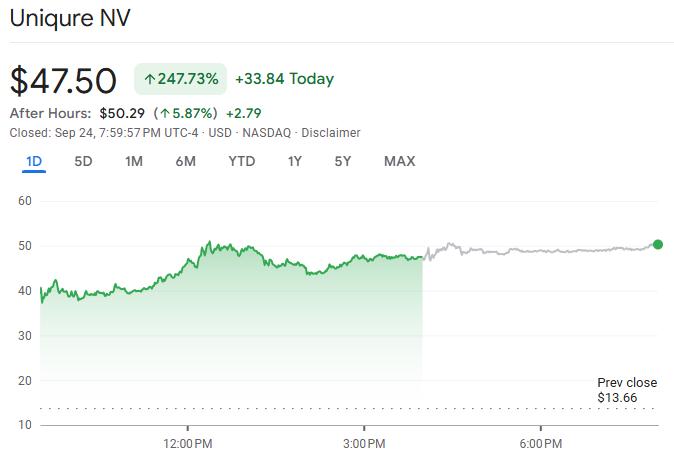
uniQure's share price rose about 247% on 24 September 2025 after positive topline results from the pivotal Phase I/II AMT‑130 study in Huntington's disease were released, which is one of the largest single‑day moves in biotech this year.
The jump reflected unusually large effect sizes at 36 months and renewed expectations for a U.S. filing in the first quarter of 2026 with the potential for a late‑2026 launch if approved.
A follow‑on financing announced the same day added a normal near‑term supply overhang that markets often need time to absorb. [1]
Trial Snapshot
These are the pivotal endpoints that drove the move and now anchor the regulatory discussion.
| Endpoint |
Result |
Window |
Comparator |
| cUHDRS (primary) |
~75% slowing of disease progression at 36 months |
36 months, data cutoff 30 June 2025 |
Propensity-matched external control from Enroll-HD |
| Total Functional Capacity (key secondary) |
Statistically significant 60% slowing at 36 months |
36 months |
Propensity-matched external control |
| CSF Neurofilament Light (biomarker) |
Mean levels below baseline at 36 months |
36 months |
Baseline within treated cohort |
| Safety |
Generally well-tolerated with a manageable profile |
Study period |
Study population |
| Press Release Timestamp |
24 September 2025 (GlobeNewswire) |
Publication date |
Company announcement |
QURE Stock's Key Numbers
Here are the headline figures investors are watching now.
| Item |
Figure |
Context |
| One-day move |
~247% on 24 Sep 2025 |
Single-session surge on topline AMT-130 data |
| cUHDRS effect |
~75% slowing at 36 months |
High-dose cohort vs propensity-matched external control |
| TFC effect |
Statistically significant 60% slowing at 36 months |
Functional capacity change vs external control |
| Biomarker |
CSF NfL below baseline at 36 months |
Directionally supportive of reduced neuronal injury |
| Regulatory timing |
BLA targeted for Q1 2026 |
Potential U.S. launch later 2026 if approved |
| Financing |
$200M offering with up to 15% over-allotment on 24 Sep 2025 |
Underwritten follow-on commenced post-rally |
Guidance & Timeline
These dates and decisions frame the next leg of the story and will shape whether price can revisit the spike.
FDA interaction: Management aims to meet the FDA before filing to align on endpoints, external controls, and review mechanics. [2]
BLA submission: Targeted for the first quarter of 2026, setting up a potential late‑2026 U.S. entry if approved.
Financing milestones: Final terms and any exercise of the 15% overallotment will determine near‑term supply absorption in the market.
What Could Re‑Test QURE Stock's Highs

A retest for QURE stock is more likely if three things line up: smooth offering execution with strong demand, constructive FDA feedback that validates the external‑control approach, and steady confidence that the 36‑month efficacy and safety signals are durable as more details are discussed with reviewers.
Those conditions would reduce uncertainty while preserving the core bull case built on clinical effects that are unusually large for this disease area.
1. Financing Read‑Through
A $200 million raise is typical for gene therapy programmes that are preparing for manufacturing scale‑up and launch, and the supply effect often fades once pricing and allocations are clear and the book is absorbed.
The dilution is real in the short run, but proceeds can fund manufacturing, access work, and commercial readiness, which strengthens the medium‑term launch plan if the therapy is approved.
2. Regulatory Path
The 36‑month data show a statistically significant 75% slowing on cUHDRS and a statistically significant 60% slowing on TFC, both versus a propensity‑matched external control, which is notable in a condition with no approved disease‑modifying therapies.
The use of external controls can invite detailed scrutiny, so alignment on evidentiary standards, potential review designations, and the scope of any post‑marketing commitments will be decisive for timing and labelling.
Reports note that TFC has been valued by the FDA in prior Huntington's assessments, which can help frame the review and label discussion if the totality of evidence is supportive.
3. Efficacy, Function, and Patients
cUHDRS captures motor, cognitive, and functional domains, so the reported slowing suggests preservation of daily capabilities if reproduced in broader use beyond the trial.
The 60% slowing on TFC addresses independence and daily living, which patients and clinicians consider highly meaningful when evaluating long‑term benefit in Huntington's disease.
Together with the biomarker direction in CSF NfL, the clinical readouts provide a coherent signal that supports a disease‑modifying claim for regulatory dialogue.
Market Mechanics and Volatility
Spikes of this size generate positioning churn and elevated volatility for days or weeks as holders rotate and new demand stabilises the tape, especially around a follow‑on financing.
Once the offering overhang clears and use of proceeds is understood, price action tends to refocus on regulatory milestones, manufacturing readiness, and payer groundwork that shape launch timing and early uptake.
Risks & Overhangs
Key constraints that could cap near‑term upside for QURE stock are set out below for clarity and focus.
Dilution and float: The offering increases share count and can weigh on the price until absorbed by demand.
Regulatory scrutiny: External‑control designs may require more justification or commitments, which can extend the process.
Manufacturing readiness: Gene therapy scale‑up requires exacting quality controls that can add timing risk.
Payer access: One‑time therapies face complex value assessments that may slow early uptake even after approval.
Competitive timelines: Peer data or shifting standards could alter the approval window or influence label scope.
Near‑Term Catalysts
Events that could shift conviction on a retest are summarised here to aid monitoring and preparation.
Offering outcome: Pricing, coverage quality, and any over‑allotment exercise will shape the near‑term supply picture.
FDA feedback: Clarity on endpoints, external controls, and potential designations would reduce regulatory uncertainty.
Additional disclosures: Durability, safety, or methodology detail that reinforces confidence into filing can support the case.
Manufacturing updates: Evidence of launch‑ready capacity, including process controls and quality milestones, can de‑risk execution.
What Could Re‑Rate the Story
Beyond a retest of highs, durable re‑rating would likely require visible progress on FDA alignment, firm manufacturing timelines, payer groundwork to shorten time‑to‑therapy after approval, and early validation of commercial infrastructure, each of which reduces perceived risk and supports higher long‑term value.
Bottom Line
The data set is strong, the timetable is defined, and the financing is purposeful, which together make a retest of the spike plausible for QURE's stock once the offering clears and the FDA dialogue is constructive.
The balance in the near term is between powerful 36‑month clinical signals, including 75% cUHDRS and 60% TFC slowing, and the normal overhangs from dilution and detailed regulatory review in a first‑of‑its‑kind setting. [3]
Disclaimer: This material is for general information purposes only and is not intended as (and should not be considered to be) financial, investment, or other advice on which reliance should be placed. No opinion given in the material constitutes a recommendation by EBC or the author that any particular investment, security, transaction, or investment strategy is suitable for any specific person.
Sources
[1] https://www.globenewswire.com/news-release/2025/09/24/3155843/0/en/uniQure-Announces-200-Million-Proposed-Public-Offering.html
[2] https://www.biopharmadive.com/news/uniqure-huntington-gene-therapy-study-results-fda/760987/
[3] https://www.biopharmadive.com/news/uniqure-huntington-gene-therapy-study-results-fda/760987/



























